Do people that recover from COVID-19 become immune to it? If so, how long does immunity to COVID-19 last?
COVID-19 Studies
Here are two recent studies have looked at this question:
- Long, April 2020 showed that 100% of cases (N=47) were immune 19 days after COVID-19 symptom onset. This is based on measuring levels of IgG antibodies, with immunity, or a positive result, defined as being above a set threshold level.
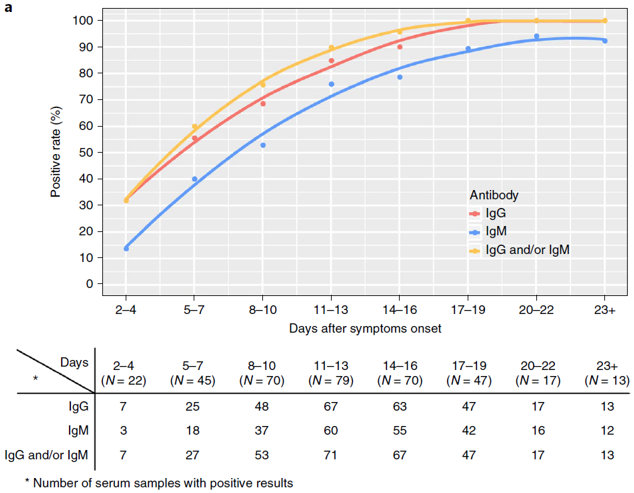
- Seow, July 2020 was a longer study, widely reported (incorrectly) as showing that immunity is short-lived. It looked at 65 individuals that were admitted to hospital and tested positive for SARS-CoV-2 (the virus linked to COVID-19). They measured three different types of IgG, relating to three parts of the virus: the surface spike protein (S), the receptor binding domain (RBD), and the nucleocapsid protein (N). For each type of IgG, 90%-95% of subjects were above threshold after 2 months. In the few cases for which they had data beyond 2 months, it appeared that IgG levels were decreasing, but this results was not statistically significant and did not reduce the immunity level.
The evidence we have so far on SARS-CoV-2 suggests that immunity after recovery from COVID-19 is almost guaranteed for at least 3 months, but beyond that it is too soon to tell.
Other Coronavirus Studies
SARS-CoV-2 is one of seven coronaviruses. The others are:
- SARS-CoV-1 (2002) – 8096 cases, 774 deaths (~10% CFR), mostly in China and Hong Kong, all in a single outbreak in 2003
- MERS-CoV (2012) – 2506 cases, 862 deaths (~35% CFR), mostly in Saudi Arabia (2014) and South Korea (2015), with minor sporadic outbreaks continuing up to 2020
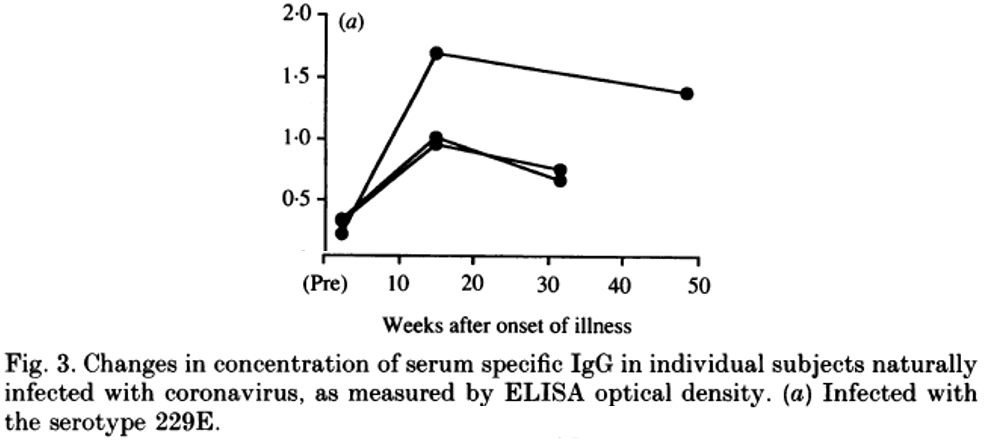
- HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1, four viruses that are found in about 15% of cases of common cold (the majority of colds are caused by rhinoviruses), with mild symptoms and no deaths.
Studies of these other coronaviruses can give us clues about COVID-19 immunity duration:
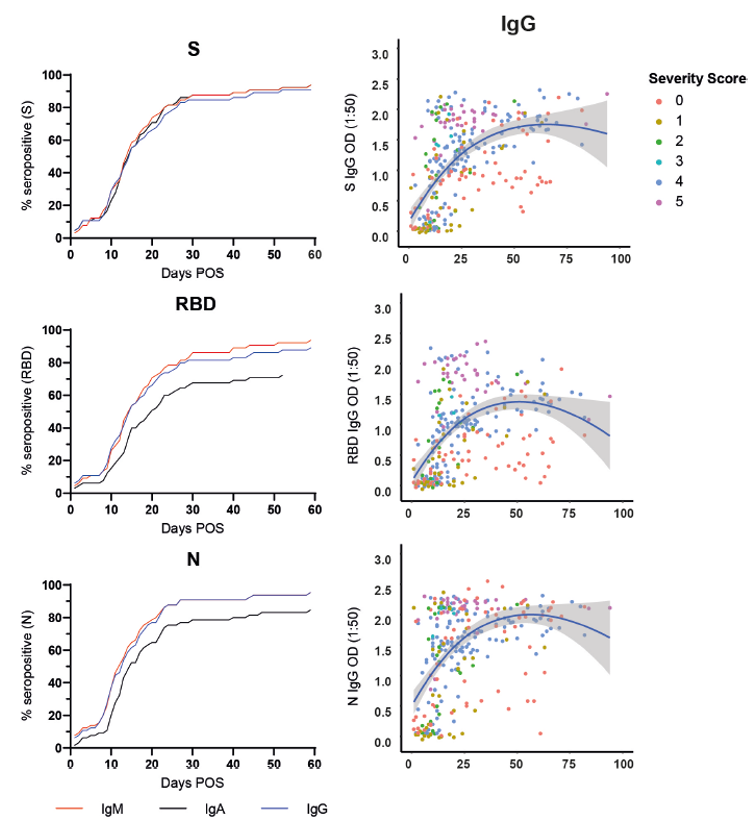
- Wu, 2007 showed that IgG antibody levels for SARS-CoV-1 were above the immunity threshold within 3 weeks in >90% of cases that had recovered from SARS, and after 3 months, 100% were immune. At 7 months it started decreasing, dropping below 90% after 2 years, and 55% after three years.
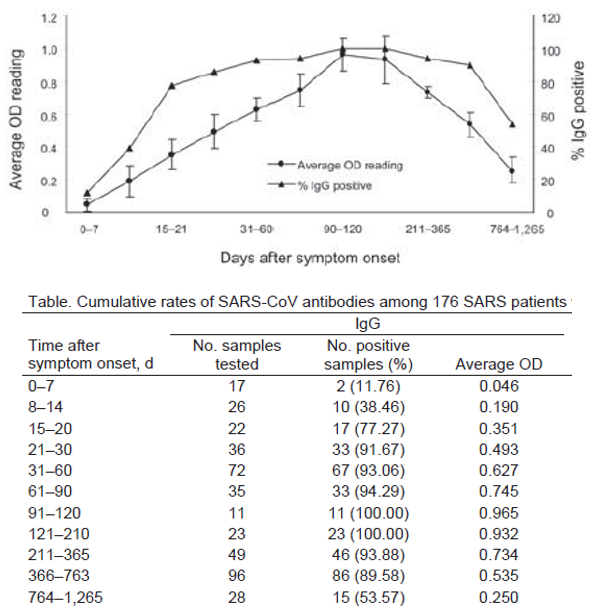
- Payne, 2016 showed that 7 out of 7 patients that recovered from MERS were still immune after 34 months, based on their antibody levels, with 5 of them seeing no decrease in antibody level.
- Callow, 1990 gave 10 volunteers a nasal spray of HCoV-229E, giving them an infection and a cold, triggering an adaptive immune response and the development of antibodies. 1 year later, the volunteers still had a high level of antibodies, and when given the same spray again did not get any cold symptoms.
Immunity is more than Antibody Levels
All the above studies measure antibody levels and then compare the measurements to a set threshold level, to determine immunity. This is a convenient but flawed way of defining and determining immunity. Merriam-Webster defines immunity as “a condition of being able to resist a particular disease especially through preventing development of a pathogenic microorganism or by counteracting the effects of its products” and immune as “having a high degree of resistance to a disease”. This definition is the common usage.
Then there is the more specific medical definition of immune, which is “having or producing antibodies or lymphocytes capable of reacting with a specific antigen”. This narrower definition is easy to measure, but it can be misleading when the two definitions are conflated, because antibodies are only a small part of our arsenal of resistance against pathogens.
The Innate Immune System
Our first line of resistance against pathogens, besides physical barriers like the lungs, gut and skin, is the innate immune system, which involves:
- Complement: proteins synthesised to identify pathogens and tag them, attack pathogen cell membranes, induce inflammation, and attract phagocytes to the infection site.
- Inflammation: changes in the blood vessels to allow phagocytes to enter tissue in the infected area more easily, and to try to prevent the infection from spreading to the rest of the body.
- Phagocytosis: innate immune system cells – macrophages, neutrophils, and dendritic cells – engulf and break down pathogens or particles. Natural Killer cells, a type of lymphocyte, target and destroy compromised host cells.
The innate immune system alone is enough to defeat most pathogens. Small infections can be cleared quickly and with only mild symptoms, if any. If we take immune to mean “having a high degree of resistance to a disease”, then having a strong innate immune system is the most important way of being immune.
The Adaptive Immune System
The adaptive immune system evolved much later than the innate immune system. Only vertebrates have an adaptive immune system. It is an add-on that exists to target specific pathogens that are causing a particularly bad infection. It involves two new types of lymphocyte, called T cells (because they mature in the thymus) and B cells (bone marrow):
- T cells become specific through the process of antigen-presentation from a dentritic cell or macrophage, and then target and destroy that pathogen, in a similar way to Natural Killer cells.
- B cells become specific through direct activation by a pathogen, and then produce antibodies to tag that pathogen, to assist the innate immune system in clearing the infection.
The adaptive immune system can take days to be activated due to the clonal selection process used to develop lymphocytes that can target the specific invading pathogen, never before encountered. After an infection has been cleared, some T cells and B cells become memory cells, adding to the immunological memory bank. Should the same pathogen return, the memory cells are activated, skipping the clonal selection process, so the adaptive immune system gets activated hours, instead of days.
Memory Cell Studies
It is the presence or absence of memory cells that determines whether the adaptive immune response will take hours or days to be activated. Antibodies may continue to circulate for months or years after an infection is cleared, but they decline if their target pathogen does not return. A person can have no antibodies, but still have memory cells, so an antibody-level test would declare them non-immune. Thus, antibody-testing does not even accurately test for the medical definition of immune.
A more direct way of assessing whether a specific pathogen is in the immunological memory bank is to test for a response from memory T cells. The following studies of are based on testing for SARS-CoV-1 memory T cells in survivors of SARS:
- Ng, 2016 demonstrated that SARS-specific memory T cells persist in three SARS-recovered individuals at 9 and 11 years post-SARS in the absence of antigen
- Le Bert, 2020 showed that memory T cells for SARS-Cov-1 remained in blood 17 years . These subjects can therefore still mount an quick adaptive immune system response to SARS-Cov-1, even if there are no measurable antibodies after so many years. The same study showed that SARS-Cov-2 is sufficiently similar to SARS-Cov-1 that an adaptive immune response even takes place in individuals that recovered from SARS 17 years ago.
Conclusion
SARS and MERS had very high Case Fatality Rates (10% and 35% respectively) and appear to confer immunity lasting at least a few years, if not decades. Common cold coronaviruses may confer immunity of a year or more, but not for as long as the more serious coronaviruses.
Covid-19 was initially said to have a CFR of 5% but this has been revised down to 0.26%, and is likely to be revised down again to <0.1%. Thus, it seems likely that SARS-Cov-2 will fall in the middle of the coronavirus duration-of-immunity spectrum.
These studies combine to show that the length of immunity following an infection is closely related to how severe the infection was, which makes sense given the evolved design of the immune system.
On an individual level, as shown by the Seow 2020 study, SARS-Cov-2 antibody levels are related to the severity of Covid-19 symptoms; the more severe the case, the stronger the immune response, the higher the antibody levels, and presumably the longer immunity lasts.
Thus, Covid-19 immunity probably lasts for years, rather than months, and longer in those that had severe symptoms. It will be shorter in those that had mild or no symptoms, whose innate immune systems were sufficient to end the infection.
